The intense blue color that iodine gives in contact with starch was discovered over 200 years ago and is a standard presence in many chemical, biochemical or medical analysis protocols – including countless titration procedures in analytical (bio)chemistry for substances as varied as vitamin C in plants extracts, dissolved oxygen concentration, or the concentration of chlorine in swimming pool water, to the activity of enzymes (e.g., amylase, an essential digestive enzyme in the human body but also a key enzyme in plants), or the detection of perspiration on skin in the so-called Minor test. Not surprisingly then, the iodine-starch reaction is a standard presence in most biochemistry textbooks.
Yet, some confusion persists when one looks at the various explanations proposed at molecular level for this reaction and for the ensuing blue color. It is generally accepted that the reaction involves the main soluble component of starch, namely amylose. Amylose is a polymer composed of glucose units/monomers, with an interesting three-dimensional structure that entails a helix with a narrow internal cavity. It is also generally accepted that a blue color occurs when iodine enters this cavity inside the amylose helix. According to some textbooks, the blue color is a result of neutral I2 molecules/units aligning themselves linearly as an infinite chain inside the central cavity of the amylose helix. By contrast, other textbooks point out that the starch-iodine reaction only happens when iodide (I-) is also present. Indeed, while iodine is normally accessible as a di-atomic unit/molecule, I2, this molecule is almost insoluble in water. To make I2 dissolve in water, one may add iodide ions, I-; these ions bind to the neutral I2 molecule, yielding mainly an anion, I3-, which (as any anion would be) is very much water-soluble. It has therefore been proposed that aligned inside the amylose helix are not chains of neutral I2 molecules, but rather chains of I3- ions. Variations on these two themes have also been proposed, that would involve structures as diverse as I4-, I5-, I7-, I9-, I62-, I82-, I102-, I42-, I6-, or I242- inside of the amylose helix.
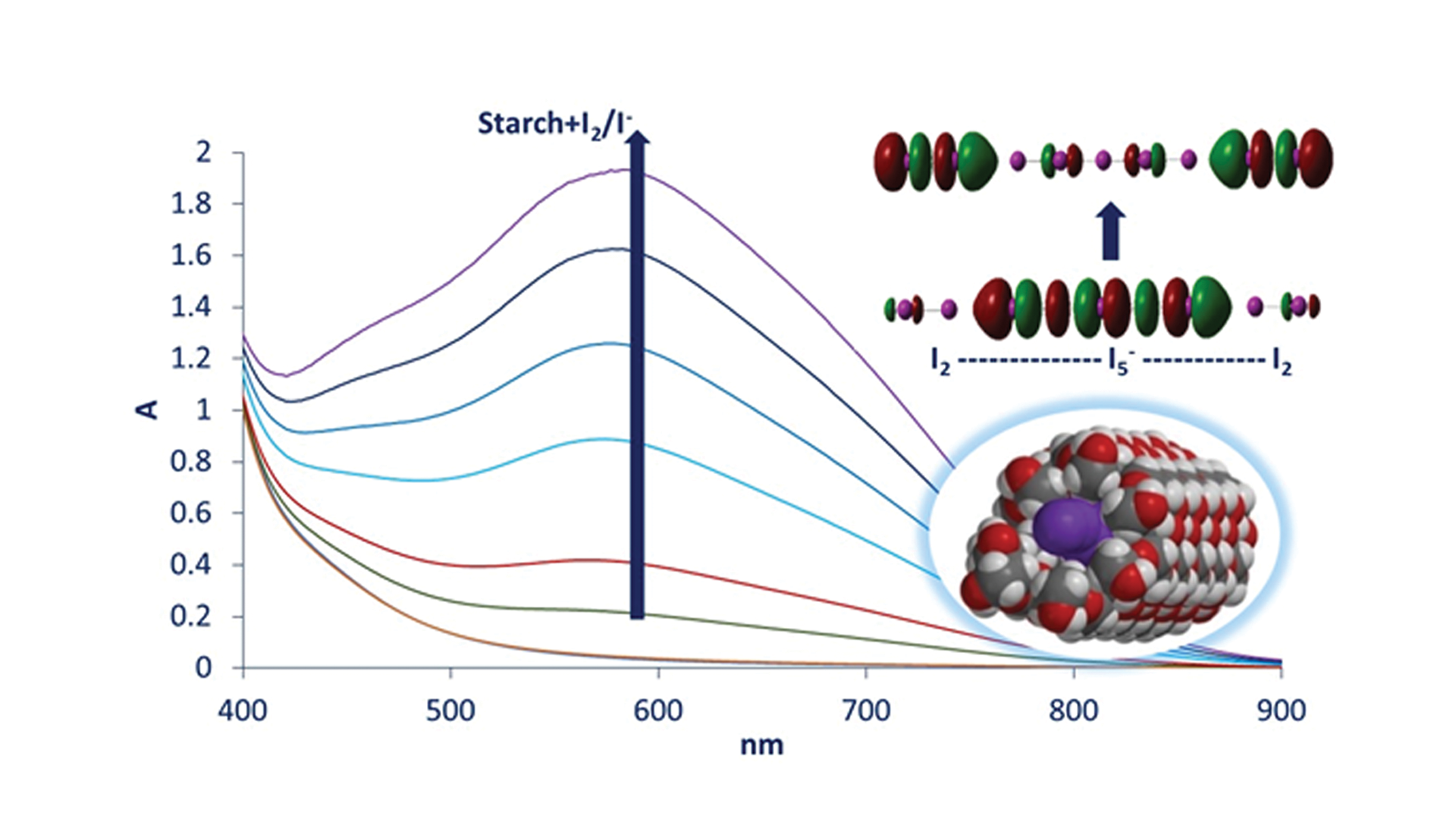
Szilárd Pesek, a PhD student at the Doctoral School of Chemistry of the Babeș-Bolyai University in Cluj-Napoca and Principal at the Leöwey Klára Highschool in Sighetu Marmației, has now employed advanced electronic structure calculations (time-dependent density functional theory, TD-DFT), alongside UV-vis spectroscopy, to try and differentiate the light-absorbing properties of various putative amylose-iodine/iodide complexes that had previously been proposed in the literature. He then compared these properties with the experimental data that he had collected, as well as with the experimental data from the literature, thereby finding that:
(1) the nature of the amylose-iodine/iodide complex involves alternating sets of I2 and Ix- units, not individual chains of I2 or Ix-
(2) the electronic processes that occur when light is absorbed by the amylose-iodine complex involve a transfer of electronic charge from the Ix- (specifically, from a σ*-type molecular orbital of Ix-) to I2 (specifically, a σ*-type orbital of I2)
(3) the best candidate for the “blue complex”, based on DFT spectral simulations, is an I2-I5–I2 unit, which is expected to occur in a repetitive manner inside the amylose helix.
This research has been published online on 16.12.2022 as:
“On The Origin of the Blue Color in The Iodine/Iodide/Starch Supramolecular Complex”: Szilárd Pesek, Maria Lehene, Adrian M. V. Brânzanic, Radu Silaghi-Dumitrescu, Molecules, 2022, 27(24), 8974; https://doi.org/10.3390/molecules27248974