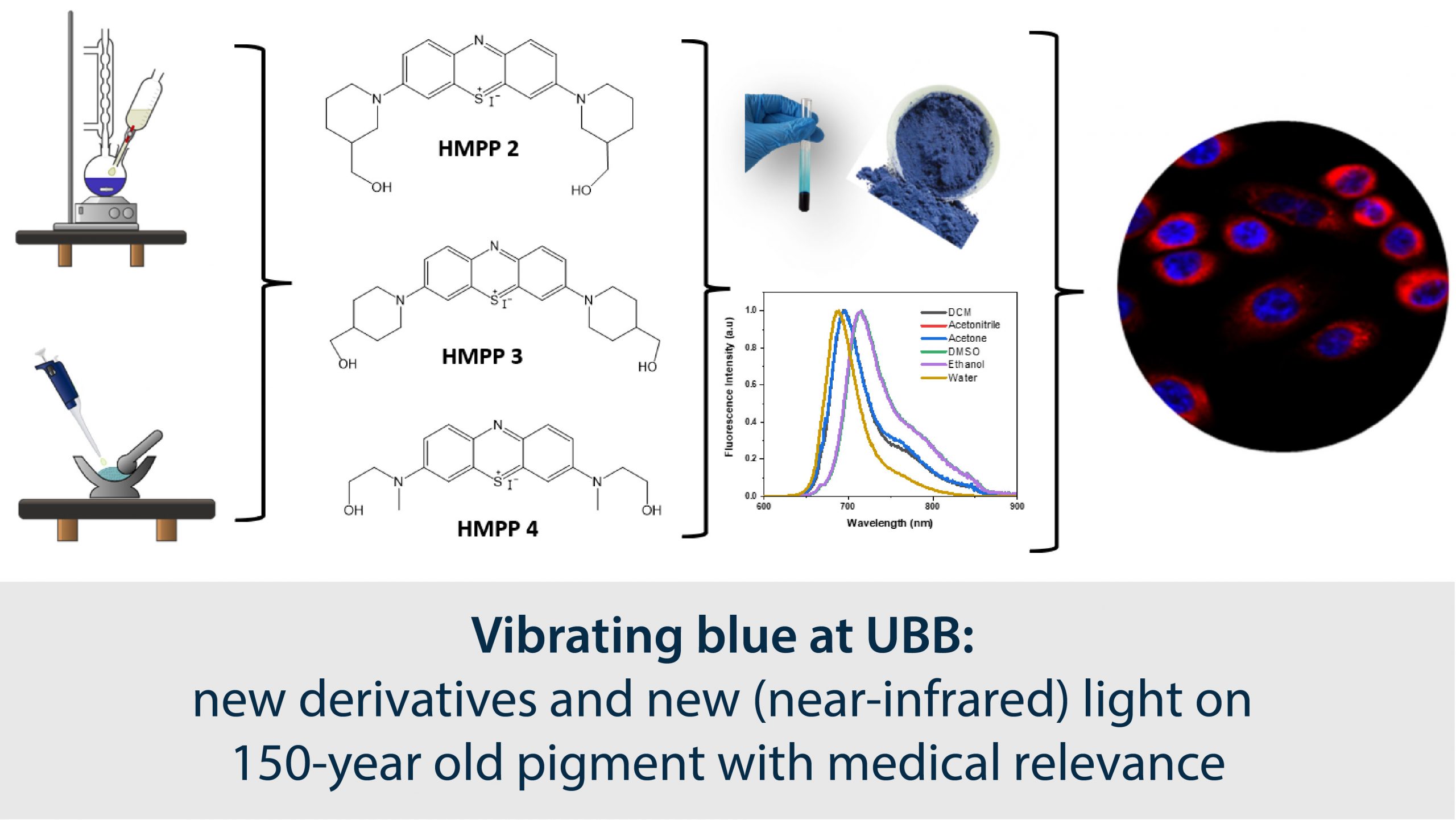
Methylene blue, a well-known substance in medicine, was first synthesized in 1876 by Heinrich Caro and has numerous applications including malaria and encephalopathy (ifosfamide-related) treatment, but also in the sensor industry as an oxygen sensor (due to its electrochemical properties). Because of the donor effect of the auxochromic groups of N,N-dimethylamine, the molecular structure of this compound allows for extended conjugation on the phenothiazine nucleus, enabling the optical properties of this compound to be extended (and useful) into the biologically-interesting near-infrared (NIR) range.
PhD student Bianca Stoean, teamed up with colleagues from the Faculty of Chemistry and Chemical Engineering, Interdisciplinary Research Institute in Bio-Nano-Sciences at UBB and with colleagues at the Institute of Oncology “Prof. Dr. Ion Chiricuță” and University of Medicine and Pharmacy Iuliu Hațieganu, has set up to synthesize new analogs of the 150-year old pigment as reported in a new article in the journal Dyes and Pigments. The new synthesized compounds in this work exhibit maximum absorption and fluorescence emission at longer wavelengths than methylene blue, which can be considered an “improvement” in the field of phenazathionium dyes.
To understand the details of how the new substituents on the methylene blue core work to affect the optical properties, the team resorted to computer modeling of the molecular structures – more specifically, density functional theory (DFT) calculations. The blue color (and the ensuing fluorescence) of this class of compounds is well understood to arise from the absorption of photons by the most reactive electrons of the respective molecule (from the so-called HOMO – the highest-energy occupied molecular orbital). However, the team’s computational results now reveal that the exact shapes and positions of the absorption/fluorescence bands and, more importantly, the major part of the intensity of the color and fluorescence, are dictated not simply by these electrons, but, to a much larger degree, by the vibrations of the molecular skeletons (i.e., the so-called vibronic contributions). This is a notable contribution, since in general the theoretical attempts to explain optical properties of molecules in chemical research often only focus on what the HOMO electrons do, and assume that the vibronic contributions are minor.
This research is available online as part of the September issue of the journal Dyes and Pigmanets as:
“New methylene blue analogues with N-piperidinyl-carbinol units: Synthesis, optical properties and in vitro internalization in human ovarian cancer cells”: Bianca Stoean, Luiza Gaina, Castelia Cristea, Radu Silaghi-Dumitrescu, Adrian M.V. Branzanic, Monica Focsan, Eva Fischer-Fodor, Bogdan Tigu, Cristian Moldovan, Andra Diana Cecan, Patriciu Achimas-Cadariu, Simion Astilean, Luminita Silaghi-Dumitrescu, Dyes and Pigments, Volume 205, 2022, 110460, https://doi.org/10.1016/j.dyepig.2022.110460 (https://www.sciencedirect.com/science/article/pii/S0143720822003825)